The Bonds in the Compound Mgso4 Can Be Described as
E the attraction between two metal atoms. Both ionic and covalent D.
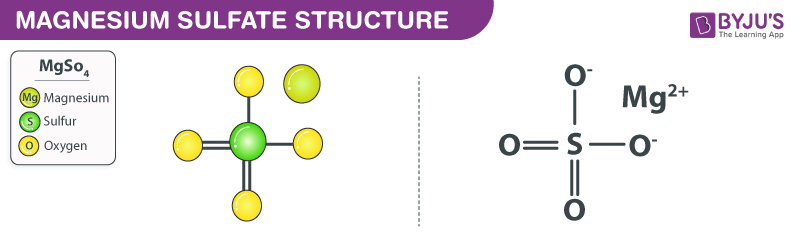
Magnesium Sulfate Mgso4 Structure Properties Uses Faqs
A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds.

. The strongest forces of attraction occur between molecules of. Here the given compound is MgSO4. What are van der waal forces in detail.
The bonds in the compound MgSO4 can be described as. Both ionic and covalent. What is chemical bond ionic bond covalent bond.
Sodium chloride is an ionic compound. Please briefly explain why you feel this question should be reported. A N02 B C02 The bonds in the compound MgS04 can be described as A neither ionic nor covalent B ionic only Which type of bond is found in sodium bromide.
Understanding the types of bonds and learning to predict shapes of molecules helps us to explain the physical and chemical of many compounds. ___ 17 The bonds in the compound MgSO4 can be described as A covalent only B neither ionic nor covalent C both ionic and covalent D ionic only ___ 18 Which compound contains both ionic and covalent bonds. The bonds in the compound MgSO4 can be described as A.
D neither ionic nor covalent. 1 Get Other questions on the subject. Science - Chemistry what are van der waal forces in detail.
What is the total number of pairs of electrons shared in a molecule of N2. In this compound Sulphur and Oxygen reacts to form covalent bond and the metal Magnesium reacts with both non metals Sulphur and Oxygen to form ionic bonds. Chemistry 21062019 1730.
Science - Chemistry A single atom of an element has 21 neutrons 20 electrons and 20 protons. Please briefly explain why you feel this answer should be reported. The bonds in compound mgso4 can be described as.
Here the given compound is MgSO4. Hence in the given compound MgSO4 there are both covalent and. The bonds in compound A are stronger than the bonds in compound B.
The oxygen atoms that are singly bonded to the sulphur atom each hold a negative charge of magnitude -1. Both ionic and covalentD. The chemical formula for nickel II bromide is.
Which formula correctly represents the composition of iron III oxide. CO32- ions contain covalent bonds. An ionic bond is formed between the magnesium cation and the sulfate anion in magnesium sulfate.
The bonds in the compound. Magnesium sulfur and oxygen. Classify The Following Compounds As Ionic Or Covalent Or Both.
Previous question Next question. B the transfer of electrons from one atom to another. The bonds in the compound MgSO4 can be described as A.
Many bonds can be covalent in one situation and ionic in another. Each atom consists of protons neutrons and electrons. Neither ionic nor covalent.
Here the given compound is MgSO4. Na Na 1 e-11p 11p 11e- 10e-Cl 1 e- Cl-17p 17p 17e- 18e-Ions. Neither ionic nor covalent Answer C.
D the attraction between two nonmetal atoms. What is the elements combined in the chemical compound represented by the formula MgSO4. In the given compound Sulphur S and Oxygen O are two non metals and Magnesium Mg is a metal.
The bonds in the compound MgSO4 can be described as A. The bonds in the compound MgSO4 can be described as Both ionic and covalent The data table below represents the properties determined by the analysis of substances A B C and D. In this compound Sulphur and Oxygen reacts to form covalent bond and the metal Magnesium reacts with both non metals Sulphur.
A metallic B ionic Which of the following solids has the highest melting point. A S02s B H20s. 2 A covalent bond is best described as A the sharing of.
The bonds in the compound MgSO4 can be described as. Which compound contains ionic bonds. 1 An ionic bond is best described as A the sharing of electrons.
C the attraction that holds the atoms together in a polyatomic ion. The bonds in the compound MgSO4 can be described as A. C both ionic and covalent.
The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic. Nano3 Ionic Or Covalent. For instance hydrogen chloride HCl is a gas in which the hydrogen and chlorine are covalently bound but if HCl is bubbled into water it ionizes completely to give the H and Cl- of a hydrochloric acid solution.
A MgF2 B CaCO3 C CH2O D PCl3 ___ 19 The data table below represents the properties determined by the analysis of substances A B C and D. Hence in the given compound MgSO4 there are both covalent and ionic bonds. In the given compound Sulphur S and Oxygen O are two non metals and Magnesium Mg is a metal.
Do you know the answer I know it Think so Line No Idea i. Na2CO3 both Na ions and CO32- ions. In this compound Sulphur and Oxygen reacts to form covalent bond and the metal Magnesium reacts with both non metals Sulphur and Oxygen to form ionic bonds.
Select all the statements that correctly described bonding in the ionic compound MGSo4. Sulfur and oxygen non metals forms a covalent bond while the magnesium a metal will react with both non metals to form an ionic bond. In the given compound MgSO4 there are both covalent and ionic bonds.
H3AsO4 is ionic compound. MgSO4 Magnesium sulfate is ionic bond. In the sulfate anion there exist two sulphur-oxygen double bonds and two sulphur-oxygen single bonds.
In the given compound Sulphur S and Oxygen O are two non metals and Magnesium Mg is a metal.

Is Mgso4 Magnesium Sulfate Ionic Or Covalent Youtube
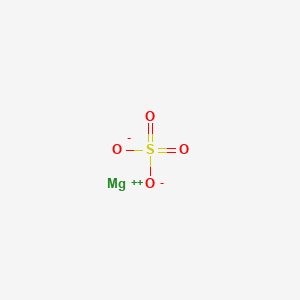
Magnesium Sulfate Mgso4 Pubchem

How To Draw The Lewis Dot Structure For Mgso4 Magnesium Sulfate Youtube
No comments for "The Bonds in the Compound Mgso4 Can Be Described as"
Post a Comment